
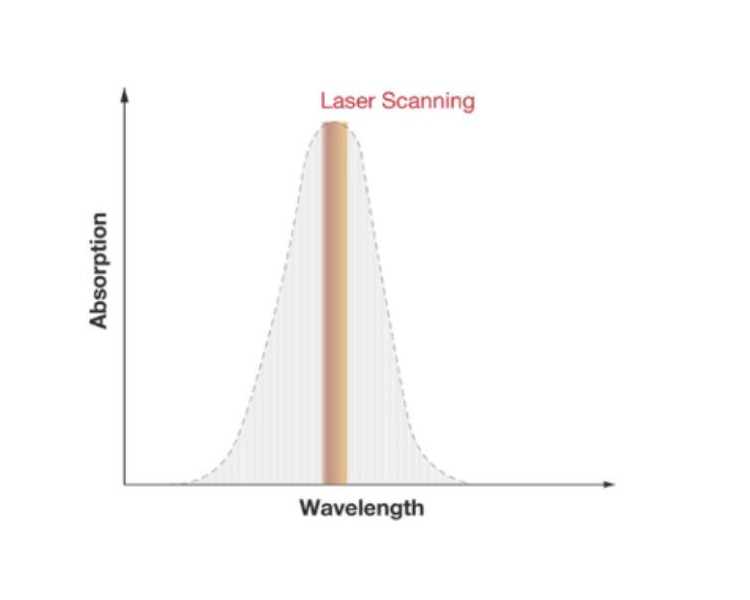
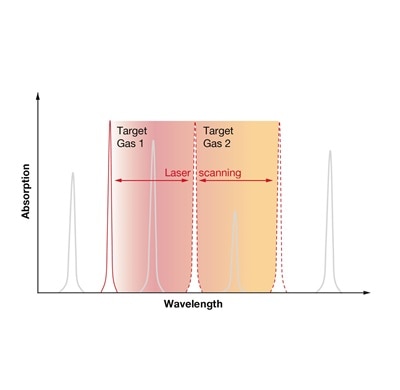
TDLS uses narrowband semiconductor lasers. Their wavelength is tuned to the absorption line of the target gas. After absorption of some photons of the target gas, the remaining light from the laser hits a photodiode. The absorption is calculated according to the Beer-Lambert law. The amount of absorption depends on the path length of the laser light in the gas cell and the absorption coefficient of the target gas. Electronic lock-in technology allows separating the gas absorption information from electro-optical system information, leading to a detection method that eliminates the need for a physical reference channel and offers continuous sensor status monitoring.
To increase the detection limit in this process, it is common to extend the path of the laser beam. State of the art multi pass cells, such as the Herriott Cell are used to reflect the laser beam multiple times between two mirrors before it hits the photodiode. Currently available solutions have path lengths of up to several meter for flow through gas cells designs. Open path approaches can can even achieve kilometre long paths.